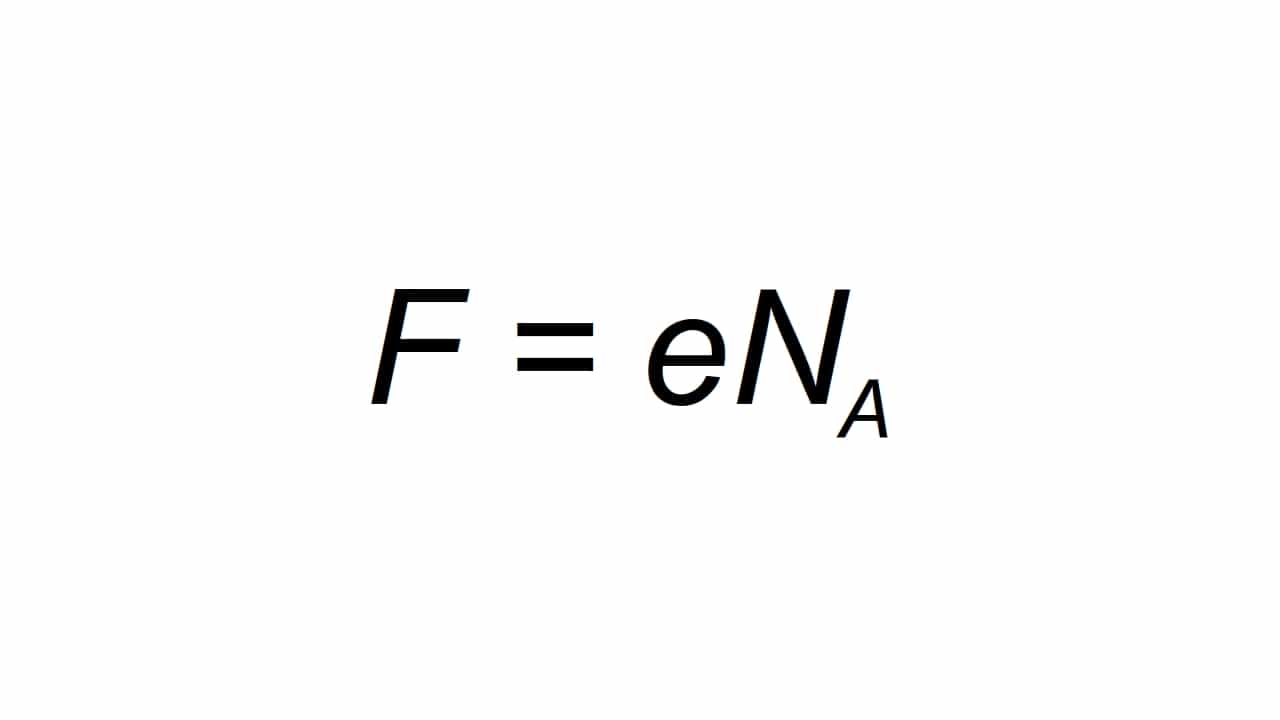As on other times we have commented on other fundamental questions in the field of electronics and electricity, such as the Ohm's law, Or the Kirchoff's laws, and even the types of basic electrical circuits, it would also be interesting to know what it is Faraday's constant, since it can help you to know a little more about the loads.
In this article you will be able to understand a little better what is constant bliss, what can it be applied for, and how is it calculated ...
What is the Faraday constant?
La Faraday's constant it is a constant widely used in the fields of physics and chemistry. It is defined as the amount of electric charge per mole of electrons. Its name comes from the British scientist Michael Faraday. This constant can be used in electrochemical systems to calculate the mass of the elements that form in an electrode.
It can be represented by the letter F, and is defined as the molar elemental charge, being able calculate on the table:
Being F the resulting value of Farday's constant, e the elemental electric charge, and Na is Avogadro's constant:
- e = 1.602176634 × 10-19 C
- Na = 6.02214076 × 1023 mol-1
According to the SI this Faraday constant is exact, like other constants, and its precise value is: 96485,3321233100184 C / mol. As you can see, it is expressed in the unit C / mol, that is, coulombs per mole. And to understand what these units are, if you don't know yet, you can continue reading the next two sections ...
What is a mole?
Un mol is a unit that measures the amount of substance. Within the SI of units, it is one of the 7 fundamental quantities. In any substance, be it an element or a chemical compound, there are a series of elemental units that compose it. One mole would be equivalent to 6,022 140 76 × 1023 elementary entities, which is the fixed numerical value of Avogadro's constant.
These elemental entities can be an atom, a molecule, an ion, an electron, photons, or any other type of elemental particle. For example, with this you can calculate the number of atoms what is in a gram of a given substance.
At Lóleo Eventos, chemistry, the mole is essential, since it allows many calculations to be made for compositions, chemical reactions, etc. For example, for water (H2O), you have a reaction 2 H2 + O2 → 2H2O, that is, that two moles of hydrogen (H2) and one mole of oxygen (O2) react to form two moles of water. Furthermore, they can also be used to express concentration (see molarity).
What is the electric charge?
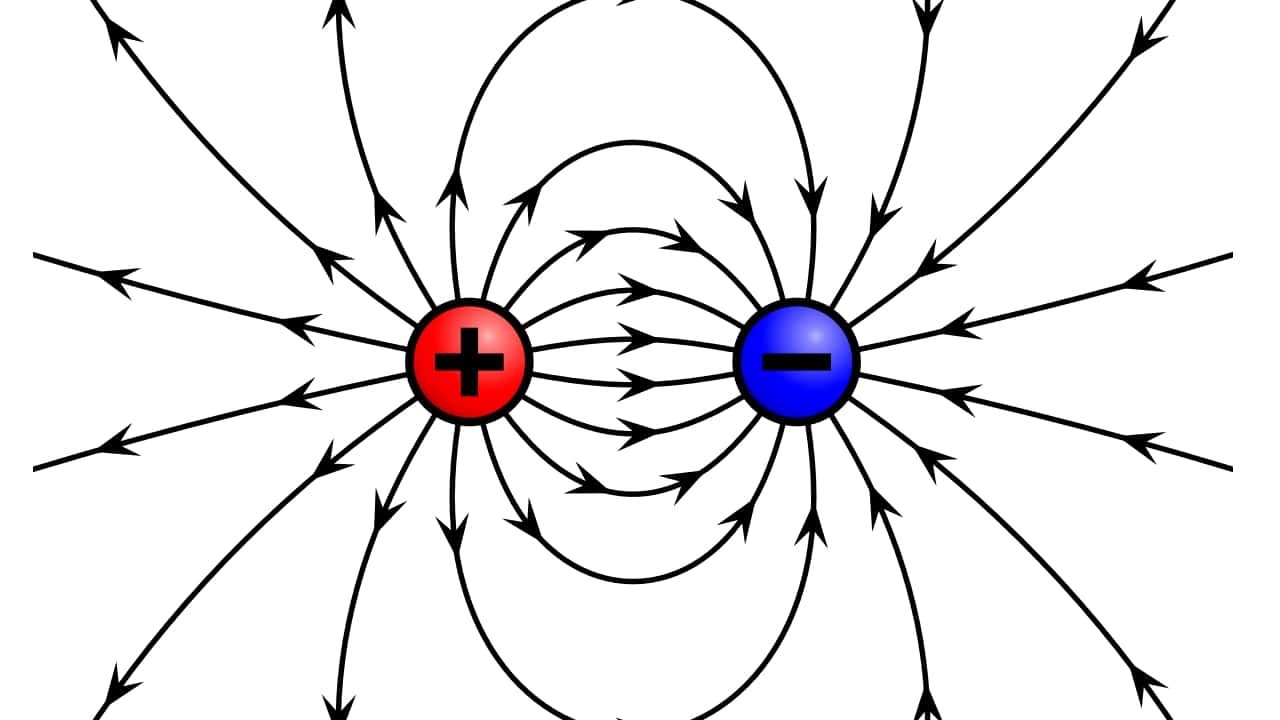
On the other hand, from the electric charge We have already spoken on other occasions, it is an intrinsic physical property of some subatomic particles that manifest attractive and repulsive forces between them due to electromagnetic fields. The electromagnetic interaction, between the charge and the electric field, is one of the 4 fundamental interactions in physics, along with the strong nuclear force, the weak nuclear force, and the gravitational force.
To measure this electrical charge, the Coulomb (C) or Coulomb, and is defined as the amount of charge carried in one second by an electric current of intensity one ampere.
Applications of the Faraday constant

If you wonder what practical application You can have this Faraday constant, the truth is that you have quite a few, some examples are:
- Electroplating / anodizing: for processes in the metallurgical industry where one metal is coated with another by electrolysis. For example, when steel is galvanized with a layer of zinc to give it greater resistance to corrosion. In these processes, the metal to be coated is used as the anode and the electrolyte is a soluble salt of the anode material.
- Metal purification: it can also be applied to the formulas used for the refinement of metals such as copper, zinc, tin, etc. Also by electrolysis procedures.
- Chemical manufacturing: to produce chemical compounds this constant is also usually used.
- Chemical analysis: by electrolysis the chemical composition could also be determined.
- Gas production: gases such as oxygen or hydrogen that are obtained from water by electrolysis also use this constant for calculations.
- Medicine and aestheticsElectrolysis can also be used to stimulate certain nerves or treat certain problems, in addition to removing unwanted hair. Without the constant, a multitude of tools of this type could not have been developed.
- Impresión: For printers, electrolysis processes are also used for certain elements.
- Electrolytic capacitors: a well-known electronic component consisting of a thin film of aluminum oxide and an aluminum anode between electrodes. The electrolyte is a mixture of boric acid, glycerin, and ammonium hydroxide. And this is how those great capacities are achieved ...
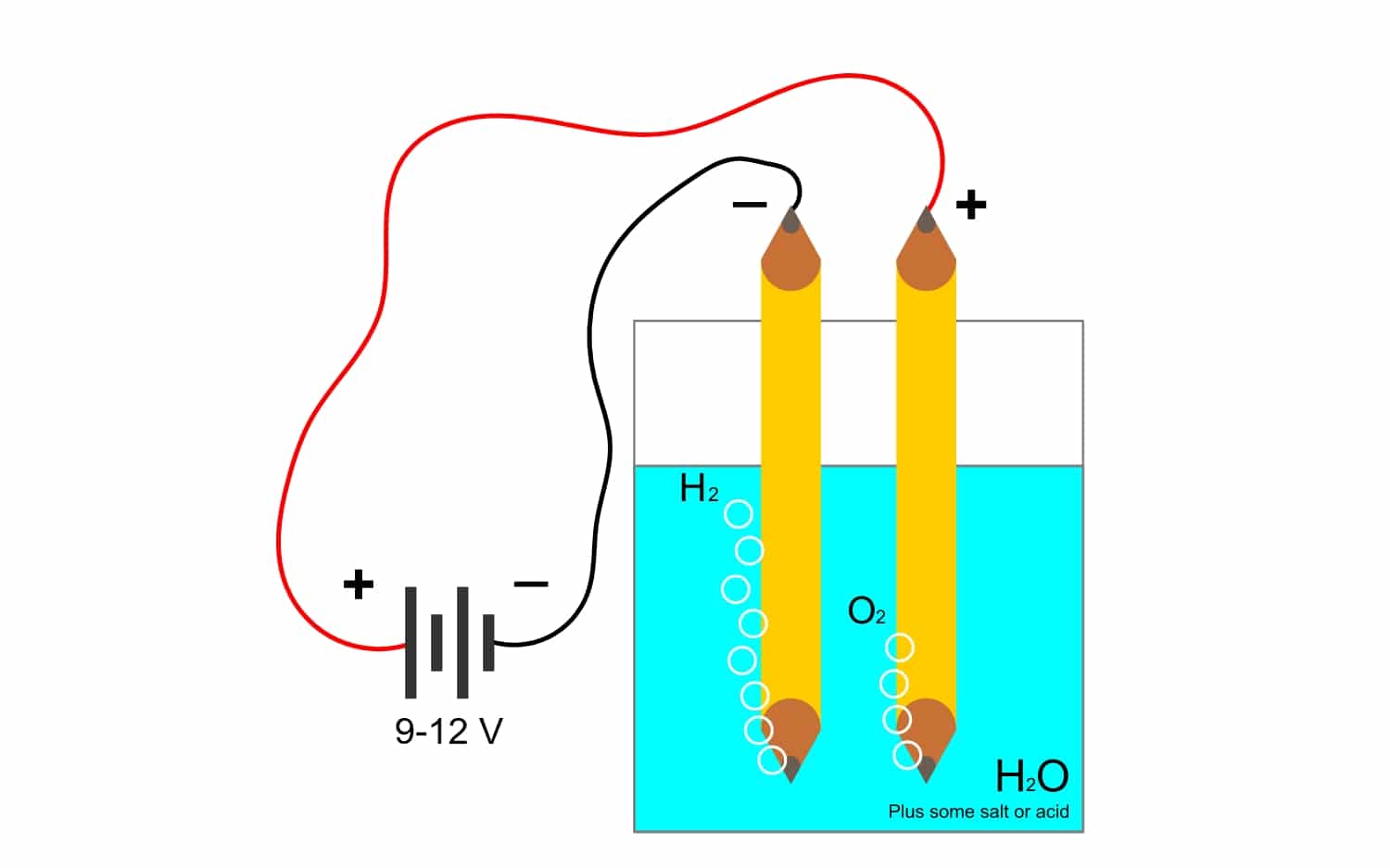
What is electrolysis?
And since the Faraday constant is so closely related to the electrolysisLet's see what this other term is that is used a lot in the industry. Thanks to this process, elements of a compound can be separated by means of electricity. This is done by the release of electrons by the anode anions (oxidation) and the capture of electrons by the cathode cations (reduction).
It was discovered accidentally by William Nicholson, in 1800, while studying the operation of chemical batteries. In 1834, Michael Faraday developed and published the laws of electrolysis.
For example, electrolysis of water H2O, allows to create oxygen and hydrogen. If a direct current is applied through electrodes, which will separate the oxygen from the hydrogen, and be able to isolate both gases (they cannot come into contact, since they produce a very dangerous explosive reaction).