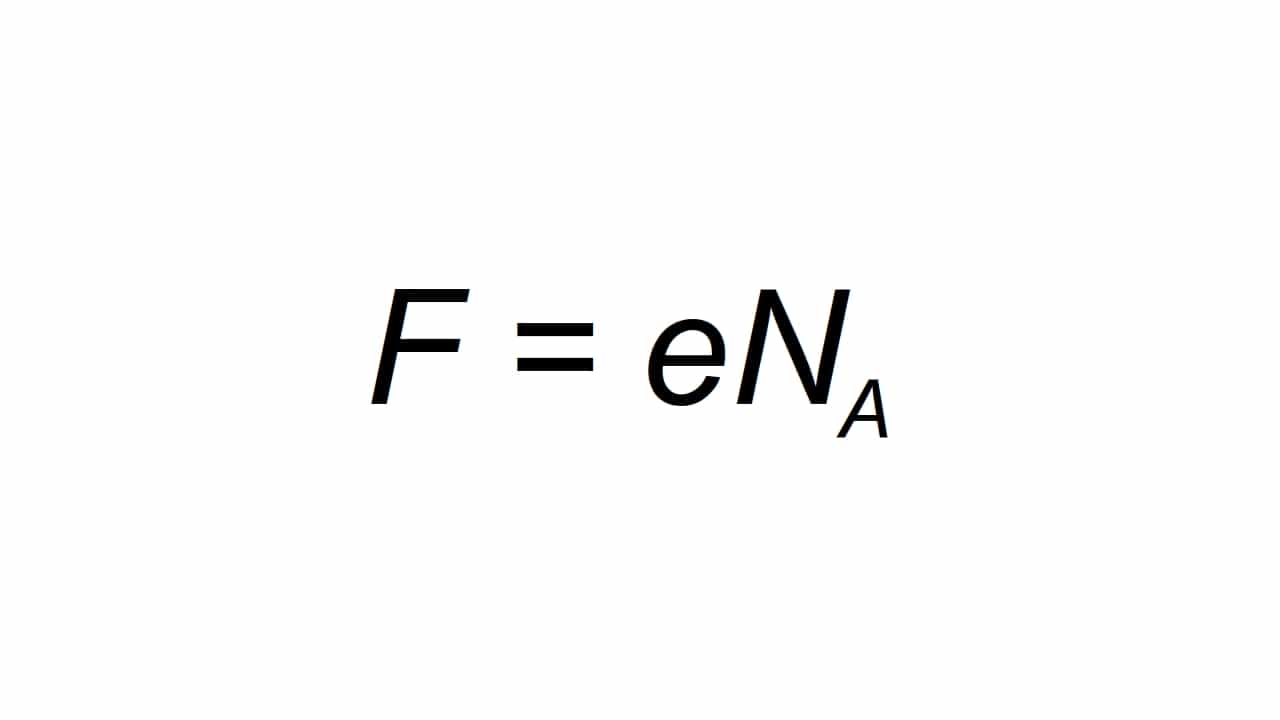Kamar sauran lokuta mun yi tsokaci kan wasu muhimman tambayoyi a fagen lantarki da wutar lantarki, kamar Dokar Ohm, raƙuman ruwa Dokokin Kirchoff, har ma da nau'ikan hanyoyin lantarki na asali, zai kuma zama abin sha’awa don sanin menene Kullum Faraday, tunda yana iya taimaka muku ƙarin sani game da kaya.
A cikin wannan labarin za ku fahimci kaɗan kaɗan menene ni'ima ta kullum, me za a iya amfani da shi, kuma ta yaya ake lissafi ...
Menene Faraday akai?
La Kullum Faraday yana da amfani sosai a fannonin kimiyyar lissafi da sunadarai. An ayyana shi azaman adadin cajin wutar lantarki ga kowane ɗigon electrons. Sunanta ya fito ne daga masanin kimiyyar Burtaniya Michael Faraday. Ana iya amfani da wannan jindadin a cikin tsarin lantarki don lissafin yawan abubuwan da ke cikin electrode.
Ana iya wakilta ta harafin F, kuma an bayyana shi azaman cajin kuzarin farko, iyawa ƙidaya kamar:
Kasancewa F sakamakon da aka samu na Farday na dindindin, e cajin wutar lantarki na asali, kuma Na shine na Avogadro:
- e = 1.602176634 × 10-19 C
- Na = 6.02214076 × 1023 tawadar Allah-1
Dangane da SI wannan madaidaicin Faraday daidai ne, kamar sauran madaidaci, kuma madaidaicin ƙimar sa shine: 96485,3321233100184 C / mol. Kamar yadda kuke gani, an bayyana shi a cikin naúrar C / mol, wato coulombs a kowace mole. Kuma don fahimtar menene waɗannan raka'a, idan baku sani ba tukuna, zaku iya ci gaba da karanta sassan biyu masu zuwa ...
Mene ne tawadar Allah?
Un tawadar Allah sashi ne wanda ke auna adadin abu. A cikin SI na raka'a, yana ɗaya daga cikin mahimman abubuwa 7. A cikin kowane sinadari, ya zama wani sinadari ko sinadarin sinadarai, akwai jerin sassan farko da suka tsara shi. Guda ɗaya zata yi daidai da 6,022 140 76 × 1023 Ƙungiyoyin farko, wanda shine ƙimar adadi na tsayayyen Avogadro.
Waɗannan ƙananan abubuwa na iya zama atom, molecule, ion, electron, photons, ko kowane nau'in barbashi. Misali, tare da wannan zaku iya lissafin adadin atoms abin da ke cikin gram na wani abu da aka ba shi.
A cikin sunadarai. Misali, don ruwa (H2O), kuna da amsa 2 H2 + Ya2 → 2H2O, wato, ɗanyen iskar hydrogen guda biyu (H2) da ɗigon oxygen ɗaya (O2) amsa don samar da ɗimbin ruwa biyu. Bugu da ƙari, ana iya amfani da su don bayyana maida hankali (duba molarity).
Menene cajin wutar lantarki?
A gefe guda, daga cajin lantarki Mun riga mun yi magana a wasu lokutan, dukiya ce ta zahiri ta wasu ƙananan ƙwayoyin da ke nuna ƙarfi da ƙyama tsakaninsu saboda filayen lantarki. Hulɗar electromagnetic, tsakanin cajin da filin wutar lantarki, yana ɗaya daga cikin muhimman mu'amaloli 4 na kimiyyar lissafi, tare da ƙarfin makamin nukiliya, ƙarfin makamashin nukiliya mai rauni, da ƙarfin nauyi.
Don auna wannan cajin wutar lantarki, da Coulomb (C) ko Coulomb, kuma an bayyana shi azaman adadin cajin da ake ɗauka a cikin dakika ɗaya ta hanyar wutar lantarki mai ƙarfi ampere ɗaya.
Aikace -aikace na Faraday akai

Idan kayi mamakin menene aikace -aikace mai amfani Kuna iya samun wannan Faraday akai, gaskiyar ita ce kuna da 'yan kaɗan, wasu misalai sune:
- Electroplating / anodizing: don matakai a masana'antar ƙarfe inda ƙarfe ɗaya ya rufe da wani ta hanyar lantarki. Misali, lokacin da galvanized karfe tare da Layer na zinc don ba shi ƙarfin juriya. A cikin waɗannan hanyoyin, ana amfani da ƙarfe da za a yi rufi azaman anode kuma electrolyte shine gishiri mai narkewa na kayan anode.
- Tsarkin ƙarfe: Hakanan ana iya amfani dashi ga dabaru da ake amfani dasu don tsaftace karafa kamar jan ƙarfe, zinc, tin, da dai sauransu. Hakanan ta hanyar hanyoyin electrolysis.
- Masana kimiyya: don samar da sinadaran sunadarai wannan galibi galibi ana amfani da shi.
- Binciken sunadarai: ta hanyar electrolysis ana iya ƙaddara abun da ke cikin sinadaran.
- Samar da iskar gas: iskar gas kamar oxygen ko hydrogen da ake samu daga ruwa ta electrolysis suma suna amfani da wannan dindindin don lissafi.
- Magunguna da kayan adoHakanan ana iya amfani da wutar lantarki don tayar da wasu jijiyoyi ko magance wasu matsaloli, ban da cire gashin da ba a so. Ba tare da dindindin ba, da ba za a iya samar da ɗimbin kayan aikin irin wannan ba.
- Ɗaukaka ayyukan: Ga masu bugawa, ana kuma amfani da hanyoyin lantarki don wasu abubuwa.
- Ƙararrawar lantarki. Electrolyte shine cakuda boric acid, glycerin, da ammonium hydroxide. Kuma wannan shine yadda ake samun waɗannan manyan ƙarfin ...
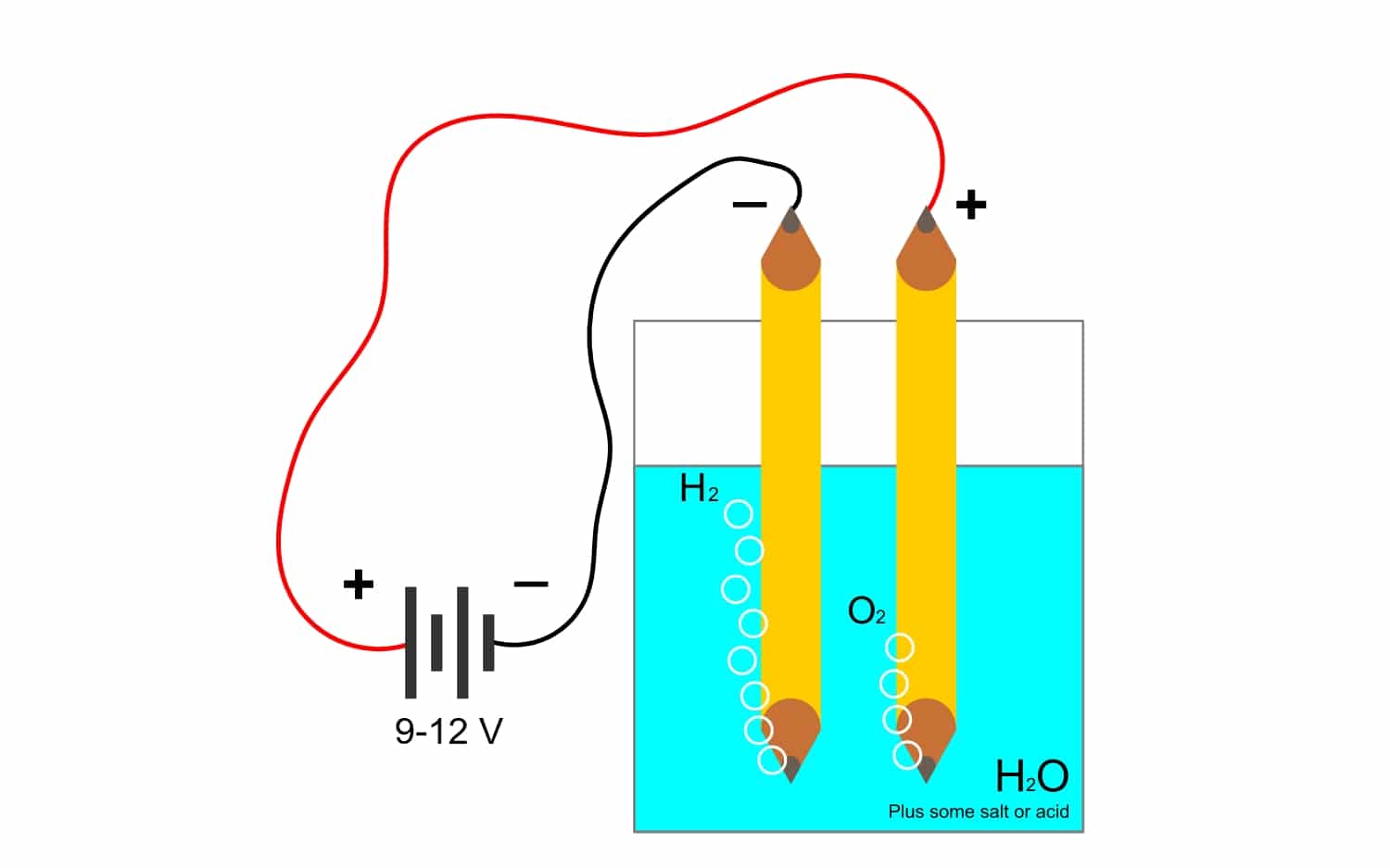
Menene electrolysis?
Kuma tunda tsayayyen Faraday yana da alaƙa sosai da lantarkiBari mu ga menene wannan sauran kalmar da ake amfani da ita sosai a masana'antar. Godiya ga wannan tsari, ana iya raba abubuwan mahadi ta hanyar wutar lantarki. Ana yin wannan ta hanyar sakin electrons ta anion anions (oxidation) da kuma kama electrons ta cathode cations (raguwa).
William Nicholson ne ya gano shi ba zato ba tsammani, a cikin 1800, yayin da yake nazarin aikin baturan sinadarai. A shekara ta 1834, Michael Faraday ci gaba da buga dokokin electrolysis.
Misali, electrolysis na ruwa H2O, yana ba da damar ƙirƙirar oxygen da hydrogen. Idan ana amfani da madaidaicin madaidaiciya ta hanyar lantarki, wanda zai raba iskar oxygen da hydrogen, kuma ya sami damar ware gas guda biyu (ba za su iya yin hulɗa da su ba, tunda suna haifar da haɗarin fashewa mai haɗari).