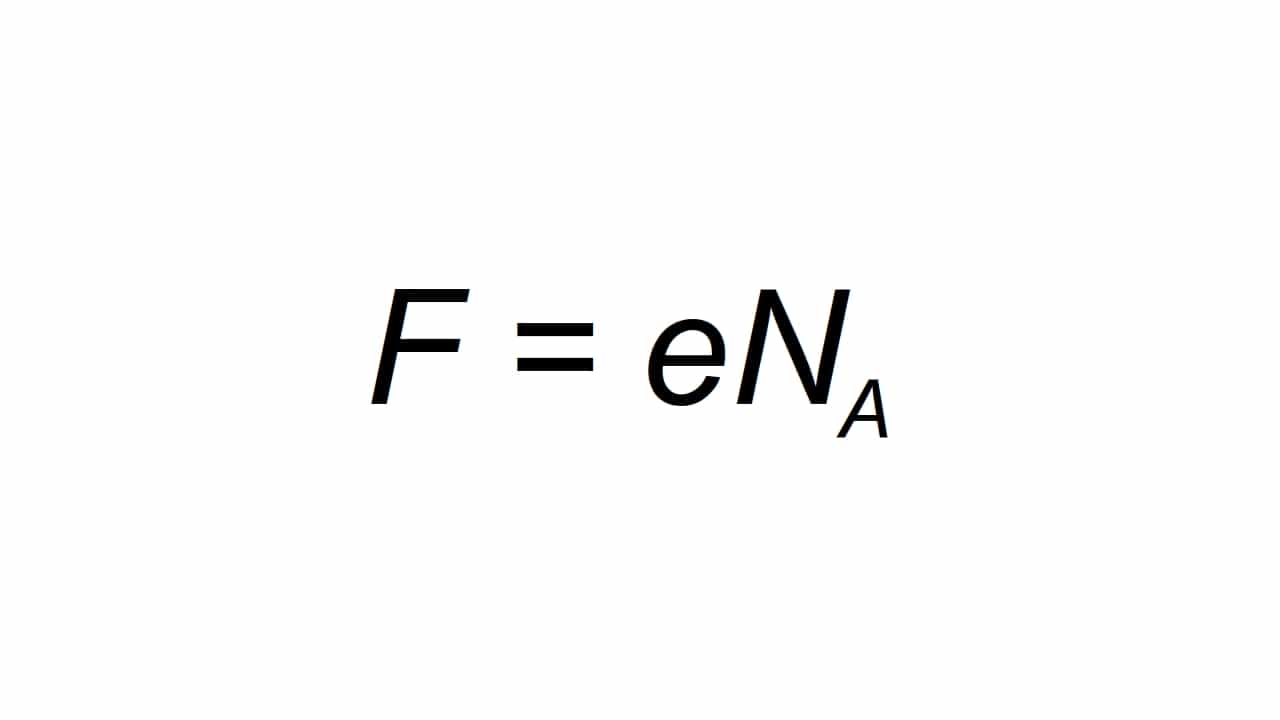Njengamanye amaxesha siye saphawula eminye imibuzo esisiseko kwicandelo le-elektroniki kunye nombane, njenge Umthetho ka-Ohm, amaza Imithetho kaKirchoff, kunye iintlobo zeesekethe zombane ezisisiseko, inokuba yinto enomdla ukuyazi ukuba yintoni Ukuhlala rhoqo kukaFaraday, njengoko inokukunceda ukwazi okungakumbi malunga nemithwalo.
Kule nqaku uza kuba nokuqonda okungcono yintoni uvuyo oluhlala luhleli, inokusetyenziselwa ntoni, kwaye ibalwa njani ...
Yintoni iFaraday engagungqiyo?
La Ukuhlala rhoqo kukaFaraday ihlala isetyenziswa ngokubanzi kwicandelo le-physics kunye ne-chemistry. Ichazwa njengesixa semali ombane ngombane kwimole nganye yee-elektron. Igama layo livela kwisazinzulu saseBritane uMichael Faraday. Oku kuhlala kunokusetyenziswa kwiinkqubo zombane ukubala ubunzima bezinto ezakha i-electrode.
Inokumelwa yileta F, kwaye ichazwa njengemali ehlawulwayo ye-molar, ekwaziyo ukubala bathanda:
Ukuba F ixabiso elibangelwayo Amaxesha onke kaFarday, e intlawulo yombane, kunye no-Na ngu-Avogadro rhoqo:
- I-e = 1.602176634 × 10-19 C
- Na = 6.02214076 × 1023 i-mole-1
Ngokwe-SI le Faraday engaguqukiyo ichanekile, njengezinye izinto ezenziwayo, kwaye ixabiso elichanekileyo yile: 96485,3321233100184 C / mol. Njengoko ubona, ibonakalisiwe kwiyunithi C / mol, oko kukuthi, ii-coulombs kwimole nganye. Kwaye ukuqonda ukuba zeziphi ezi yunithi, ukuba awukayazi okwangoku, ungaqhubeka nokufunda la macandelo mabini alandelayo ...
Yintoni i-mole?
Un i-mole Liyunithi elilinganisela ubungakanani bento. Ngaphakathi kwe-SI yeeyunithi, yenye yezinto ezisi-7 ezisisiseko. Kuyo nayiphi na into, nokuba yeyinto okanye into eyimichiza, kukho uthotho lweeyunithi ezizezayo. Imole enye iya kulingana ne-6,022 140 76 × 1023 izinto zokuqala, elixabiso elisisigxina le-Avogadro.
Ezi zinto zinokuba yi-athomu, i-molecule, i-ion, i-electron, i-photons, okanye nayiphi na into enjani. Umzekelo, ngale nto unako Bala inani leeathom yintoni kwigrama yento enikiweyo.
Kulo chemistry, i-mole ibalulekile, kuba ivumela ukubala okuninzi ukwenziwa kwengoma, ukuphendula kwamachiza, njl. Umzekelo, okamanzi (H2O), unayo impendulo 2 H2 + OKANYE2 → 2H2OOko kukuthi, iimeyile ezimbini zehydrogen (H2) kunye nemolekyuli enye yeoksijini (O2) phendula kwifom ezimbini zamanzi. Ngapha koko, zinokusetyenziselwa ukubonisa uxinzelelo (jonga ubukhulu).
Ubiza malini umbane?
Kwelinye icala, ukusuka umbane Sele sithethile ngamanye amaxesha, yipropathi yangaphakathi yomzimba wamasuntswana athile e-subatomic abonisa amandla anomtsalane kunye nonyanyekayo phakathi kwabo ngenxa yecandelo le-electromagnetic. Ukuhlangana kombane, phakathi kwentlawulo kunye nentsimi yombane, yenye yeendlela ezi-4 ezisisiseko zokusebenzisana kwi-physics, kunye namandla enyukliya, amandla enyukliya angenamandla, kunye namandla omxhuzulane.
Ukulinganisa umrhumo wombane, Coulomb (C) okanye Coulomb, kwaye ichazwa njengesixa sentlawulo esiqhutywa ngomzuzwana omnye ngumbane wombane wamandla omnye ampere.
Ukusetyenziswa kweFaraday rhoqo

Ukuba uyazibuza isicelo esisebenzayo Ungayifumana le Faraday rhoqo, inyani kukuba unembalwa, eminye imizekelo yile:
- Ukuchithwa kwamandla / i-anodizingIinkqubo kwishishini le-metallurgical apho isinyithi esinye sigutyungelwe esinye nge-electrolysis. Umzekelo, xa intsimbi ifakwa i-galvanized kunye nocwecwe lwe-zinc ukuyinika ukumelana okukhulu nokubola. Kule nkqubo, isinyithi esiza kugqitywa sisetyenziswa njenge-anode kunye ne-electrolyte yityuwa enyibilikayo yezinto ze-anode.
- Ukuhlanjululwa kwesinyithi: inokusetyenziswa kwiifomula ezisetyenziselwa ukucokisa isinyithi esifana nobhedu, zinc, tin, njl. Kwakhona ngeenkqubo ze-electrolysis.
- Ukuveliswa kwemichizaUkuvelisa iikhompawundi zemichiza eli rhoqo lihlala lisetyenziswa.
- Uhlalutyo lwekhemikhali: Ngu-electrolysis ukwenziwa kweekhemikhali nako kunokugqitywa.
- Imveliso yegesi: iigesi ezinje ngeoksijini okanye ihydrogen efumaneka emanzini nge-electrolysis nayo isebenzisa oku rhoqo ukubala.
- Amayeza kunye nobuhleI-Electrolysis ingasetyenziselwa ukukhuthaza imithambo-luvo ethile okanye ukunyanga iingxaki ezithile, ukongeza ekususeni iinwele ezingafunekiyo. Ngaphandle kwesiqhelo, ubuninzi bezixhobo zolu hlobo azinakwenziwa.
- PhrintaKwiiprinta, iinkqubo ze-electrolysis zisetyenziselwa izinto ezithile.
- Ii-capacitors ze-ElectrolyticIcandelo elaziwayo elektroniki eliqukethe ifilimu encinci ye-aluminium oxide kunye ne-aluminium anode phakathi kwee-electrode. I-electrolyte ngumxube we-boric acid, i-glycerin, kunye ne-ammonium hydroxide. Kwaye le yindlela amandla amakhulu afezekiswa ngayo ...
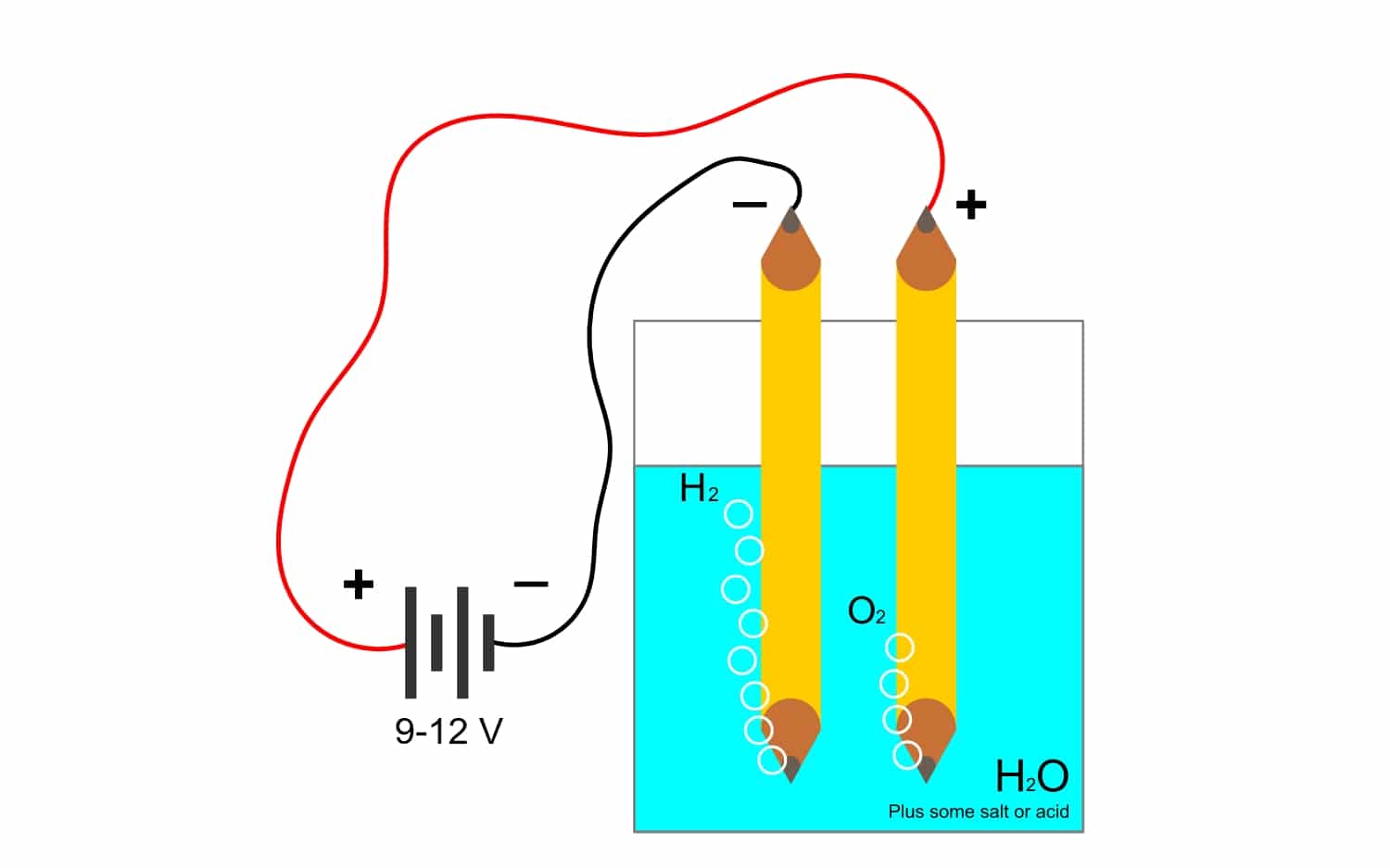
Yintoni i-electrolysis?
Kwaye okoko rhoqo kweFaraday kusondele kakhulu kunxibelelene ne- electrolysisMakhe sibone ukuba leliphi elinye igama elisetyenziswa kakhulu kushishino. Ndiyabulela kule nkqubo, izinto zekhompawundi zinokwahlulwa ngombane. Oku kwenziwa ngokukhutshwa kwee-elektroni zii-anode anion (ioksijini) kunye nokubanjwa kwee-elektroni zii-cathode cations (ukunciphisa).
Yafunyanwa ngengozi nguWilliam Nicholson, ngo-1800, ngelixa wayefunda ukusebenza kweebhetri zamachiza. Ngo-1834, UMichael Faraday iphuhlise yapapasha imithetho ye-electrolysis.
Umzekelo, i-electrolysis ye amanzi H2O, ivumela ukwenza ioksijini kunye nehydrogen. Ukuba ngoku kusetyenziswa ngokuthe ngqo ii-electrode, eza kwahlula ioksijini kwihydrogen, kwaye ikwazi ukwahlula zombini iigesi (azinakunxibelelana, kuba zivelisa indlela eyingozi).