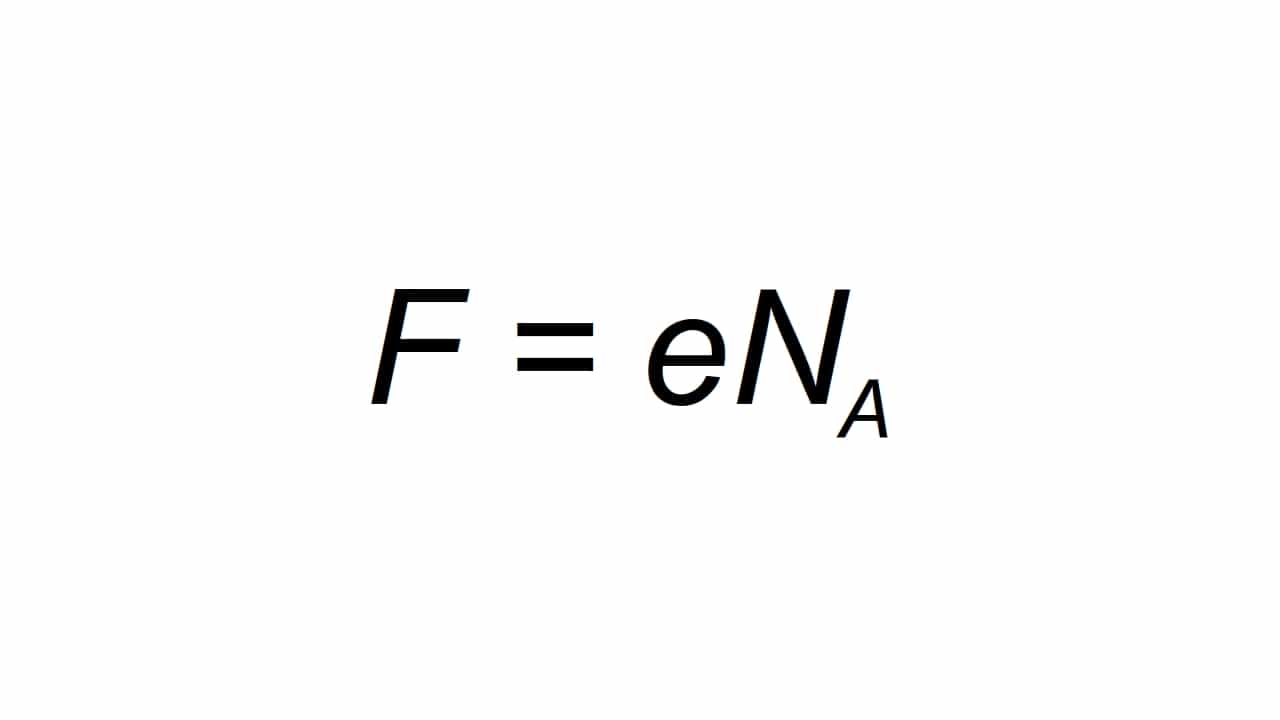Njengezinye izikhathi siphawule ngeminye imibuzo ebalulekile emkhakheni we-elekthronikhi nogesi, njenge Umthetho ka-Ohm, amagagasi Imithetho kaKirchoff, ngisho ne izinhlobo zamasekethe kagesi ayisisekelo, kungabuye kuthakazelise ukwazi ukuthi kuyini Ukuhlala njalo kukaFaraday, njengoba kungakusiza ukwazi okwengeziwe ngemithwalo.
Kulesi sihloko uzoqonda kangcono kancane yini injabulo njalo, ingasetshenziselwa ini, futhi ibalwa kanjani ...
Kuyini njalo uFaraday?
La Ukuhlala njalo kukaFaraday kuyinto esetshenziswa kabanzi njalo emkhakheni we-physics kanye ne-chemistry. Ichazwa njengenani lentengo kagesi imvukuzane ngayinye yama-electron. Igama layo livela kusosayensi waseBrithani uMichael Faraday. Lokhu kuhlala kungasetshenziswa ezinhlelweni ze-electrochemical ukubala ubukhulu bezinto ezakha i-electrode.
Ingamelwa yile ncwadi F, Futhi ichazwa njengokukhokhiswa okuyisisekelo kwe-molar, ukukwazi ukubala like:
Ukuba F inani eliholelekile Ngokuhlala kukaFarday, e amandla kagesi aphansi, kanti uNa uhlala njalo ku-Avogadro:
- e = 1.602176634 × 10-19 C
- I-Na = 6.02214076 × 1023 imvukuzane-1
Ngokwe-SI lokhu okuqhubekayo kweFaraday kunembile, njengezinye izinguquko, futhi inani laso eliqondile yile: 96485,3321233100184 C / mol. Njengoba ukwazi ukubona, ivezwa ku-unit C / mol, okungukuthi, ama-coulombs ngemvukuzane ngayinye. Futhi ukuqonda ukuthi yini lawa manyunithi, uma ungakazi okwamanje, ungaqhubeka nokufunda izigaba ezimbili ezilandelayo ...
Yini imvukuzane?
Un imvukuzane iyunithi elinganisa inani lezinto. Ngaphakathi kwe-SI yamayunithi, kungenye yezinto eziyisikhombisa eziyisisekelo. Kunoma iyiphi into, kungaba yinto noma isakhi samakhemikhali, kukhona uchungechunge lwamayunithi we-elemental ayibhalayo. Imvukuzane eyodwa ibizolingana no-7 6,022 140 × 7623 amabhizinisi aphansi, okuyinani lezinombolo elinqunyelwe lokuhlala njalo kuka-Avogadro.
Lezi zinhlangano ezingaba yi-athomu, i-molecule, i-ion, i-electron, ama-photon, noma olunye uhlobo lwenhlayiyana eyisiqalo. Isibonelo, ngalokhu ungakwazi bala inani lama-athomu yini ekugramu yento enikeziwe.
Ku chemistry, imvukuzane ibalulekile, ngoba ivumela ukubalwa okuningi ukwenziwa kokuqanjwa, ukuphendula kwamakhemikhali, njll. Isibonelo, samanzi (H2O), unokusabela I-2 H2 + NOMA2 → 2H2O, okungukuthi, ukuthi ama-moles amabili we-hydrogen (H2) kanye nemvukuzane eyodwa yomoya-mpilo (O2) usabela ekwakhiweni kwezimvukuzane ezimbili zamanzi. Ngaphezu kwalokho, zingasetshenziselwa ukuveza ukugxila (bheka ubukhulu).
Iyini imali kagesi?
Ngakolunye uhlangothi, kusuka ku- imali kagesi Sesivele sikhulumile kwezinye izikhathi, kuyimpahla engokomzimba engaphakathi yezinhlayiya ezithile ze-subatomic ezibonisa amandla ahehayo nanengekayo phakathi kwabo ngenxa yezinkambu zikagesi. Ukusebenzisana kwe-electromagnetic, phakathi kwenkokhiso nensimu kagesi, kungenye yezindlela ezi-4 zokusebenzisana okuyisisekelo ku-physics, kanye namandla amakhulu enuzi, amandla enuzi abuthakathaka, namandla adonsela phansi.
Ukukala le mali kagesi, i- Coulomb (C) noma Coulomb, futhi ichazwa njengenani lokukhokhiswa elithathwa ngomzuzwana owodwa ngamandla kagesi okuqina kwe-ampere eyodwa.
Izicelo zeFaraday njalo

Uma uzibuza ukuthi yini ukusetshenziswa okusebenzayo Ungaba nalokhu okuqhubekayo kweFaraday, iqiniso ukuthi unezimbalwa, ezinye izibonelo yilezi:
- I-Electroplating / anodizing: ngenqubo embonini ye-metallurgical lapho insimbi eyodwa imbozwa enye nge-electrolysis. Isibonelo, lapho insimbi ifakwa ngogqinsi lwe-zinc ukuyinika ukumelana okukhulu nokugqwala. Kulezi zinqubo, insimbi ezogqunywa isetshenziswa njenge-anode futhi i-electrolyte iwusawoti oncibilikayo wento ye-anode.
- Ukuhlanzwa kwensimbi: ingasetshenziswa futhi kumafomula asetshenziselwa ukucwenga izinsimbi ezifana nethusi, i-zinc, ithini, njll. Futhi ngezinqubo ze-electrolysis.
- Ukukhiqizwa kwamakhemikhali: ukukhiqiza amakhemikhali amakhemikhali lokhu kuhlala kusetshenziswa kaningi.
- Ukuhlaziywa kwamakhemikhali: nge-electrolysis ukwakheka kwamakhemikhali nakho kunganqunywa.
- Ukukhiqizwa kwegesi: amagesi afana ne-oxygen noma i-hydrogen atholakala emanzini nge-electrolysis nawo asebenzisa lokhu okungaguquki ekubaleni.
- Imithi nobuhleI-Electrolysis nayo ingasetshenziselwa ukuvuselela izinzwa ezithile noma ukwelapha izinkinga ezithile, ngaphezu kokususa izinwele ezingafuneki. Ngaphandle kokuhlala njalo, ubuningi bamathuluzi walolu hlobo abengeke athuthukiswe.
- Phrinta: Kumaphrinta, izinqubo ze-electrolysis nazo zisetshenziselwa izinto ezithile.
- Ama-capacitor kagesi: ingxenye eyaziwayo ye-elekthronikhi equkethe ifilimu elincanyana le-aluminium oxide ne-aluminium anode phakathi kwama-electrode. I-electrolyte iyinhlanganisela ye-boric acid, i-glycerin, ne-ammonium hydroxide. Futhi yile ndlela la makhono amakhulu atholakala ngayo ...
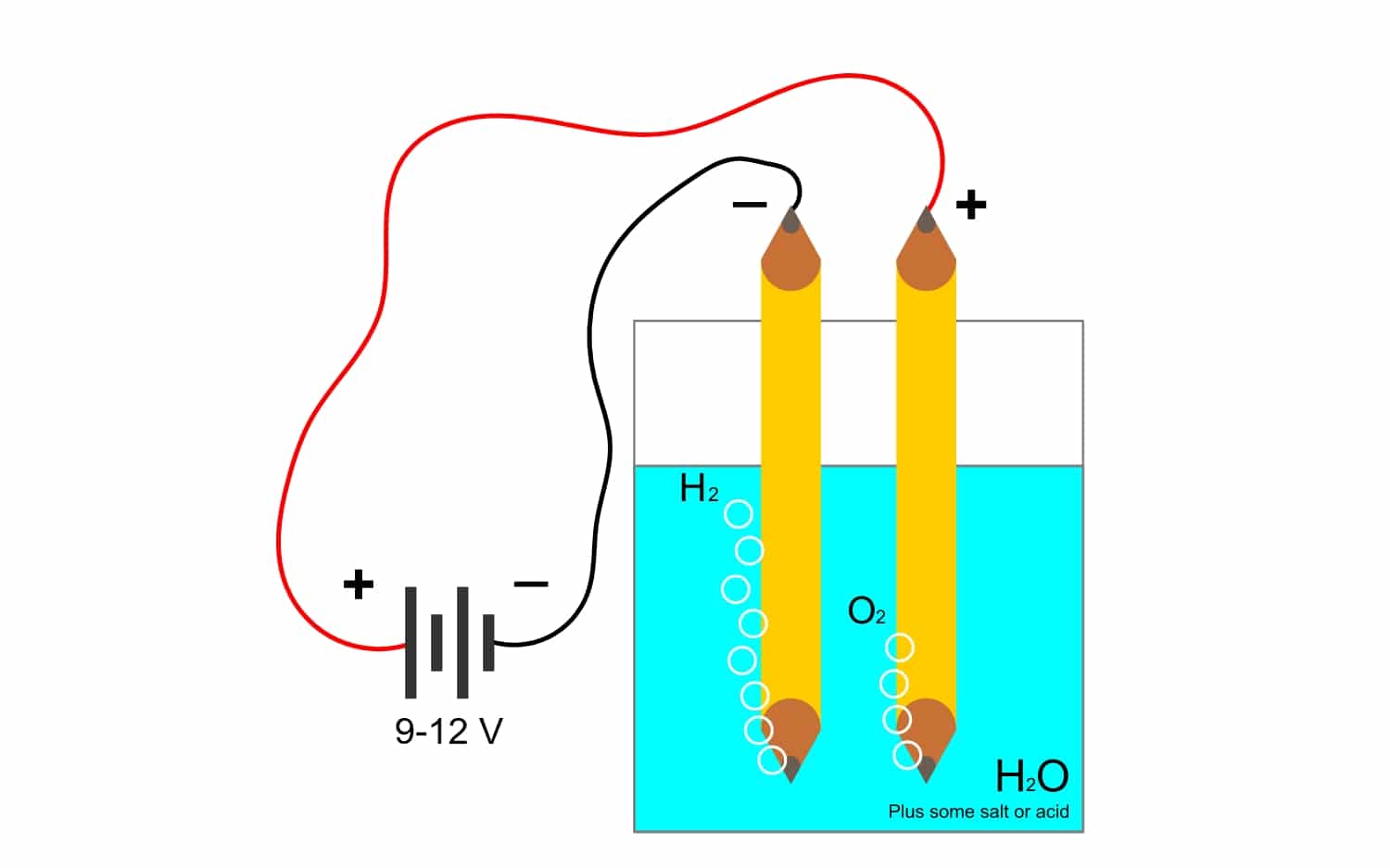
Kuyini i-electrolysis?
Futhi njengoba okuqhubekayo kweFaraday kuhlobene kakhulu ne- i-electrolysisAke sibone ukuthi yiliphi elinye igama elisetshenziswa kakhulu embonini. Ngenxa yale nqubo, izinto zekhompiyutha zingahlukaniswa ngogesi. Lokhu kwenziwa ngokukhululwa kwama-electron yi-anode anions (i-oxidation) nokubanjwa kwama-electron yi-cathode cations (ukunciphisa).
Yatholwa ngephutha nguWilliam Nicholson, ngo-1800, ngenkathi ifunda ngokusebenza kwamabhethri amakhemikhali. Ngo-1834, UMichael Faraday yathuthukisa futhi yashicilela imithetho ye-electrolysis.
Isibonelo, i-electrolysis ye- amanzi H2O, ivumela ukudala i-oxygen ne-hydrogen. Uma kusetshenziswa i-current ngqo ngama-electrode, ezohlukanisa i-oxygen ne-hydrogen, futhi ikwazi ukuhlukanisa womabili la magesi (awanakuhlangana, ngoba akhiqiza ukuqhuma okuyingozi kakhulu).